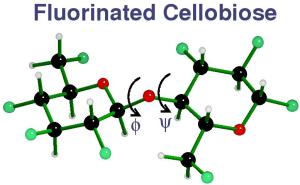
Fluorinated cellobiose and maltose as stand-ins for energy surface calculations, Tetrahedron: Asymmetry, 16 (2005) 577-586.

Fluorinated cellobiose and maltose as stand-ins for energy surface calculations, Tetrahedron: Asymmetry, 16 (2005) 577-586. |  |
A. D. French*,a, G. P. Johnsona,
A.-M. Keltererb, and G. I. Csonkac
a Southern Regional Research Center, New Orleans, LA,
USA,
b Institut für Physikalische und Theoretische
Chemie, Technische Universität Graz, Austria
c Department of Inorganic Chemistry, Budapest University
of Technology, Hungary.
Abstract:
To better understand computational predictions of disaccharide
conformations, 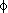 ,
,
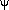 maps were constructed for two analogs in which all
hydroxyl groups were replaced with fluorine atoms (F-cellobiose
and F-maltose). These molecules do not permit hydrogen bonding
but should five better steric representation than analogs in which
hydrogen atoms replaced the exo-cyclic groups. Hartree-Fock and
B3LYP density functional quantum mechanics (QM) theory were used.
The preferred ring shape for fluorinated glucose depends on the
level of QM theory, but over the limited phi,psi space that was
studied, the rings remained in the 4C1 form. Also, fluorine atoms
are remote enough that they do not affect the torsional energies for
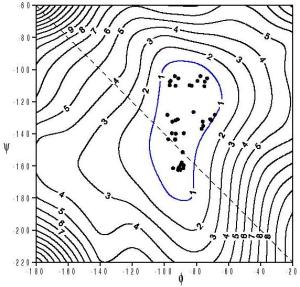
the glycosidic bonds. F-Cellobiose maps (see images) were predictive of the
conformations in crystals, but F-maltose
maps were less so. The QM F-cellobiose map and an MM4::QM hybrid
map for cellobiose itself were similar. However, the hybrid maltose
map had many more experimental conformations within its 2 kcal/mol
contour than did the QM F-maltose map. The apparent mean strength
of an intra-molecular, inter-residue hydrogen bond is about
3 kcal/mol, based on the energy for many of the hydrogen bonded
maltose sturctures on the F-maltose map. The F-maltose map was
similar to a new QM map for an analog of maltose in which all
hydroxyl groups were replaced with hydrogen atoms.
maps were constructed for two analogs in which all
hydroxyl groups were replaced with fluorine atoms (F-cellobiose
and F-maltose). These molecules do not permit hydrogen bonding
but should five better steric representation than analogs in which
hydrogen atoms replaced the exo-cyclic groups. Hartree-Fock and
B3LYP density functional quantum mechanics (QM) theory were used.
The preferred ring shape for fluorinated glucose depends on the
level of QM theory, but over the limited phi,psi space that was
studied, the rings remained in the 4C1 form. Also, fluorine atoms
are remote enough that they do not affect the torsional energies for
the glycosidic bonds. F-Cellobiose maps (see images) were predictive of the
conformations in crystals, but F-maltose
maps were less so. The QM F-cellobiose map and an MM4::QM hybrid
map for cellobiose itself were similar. However, the hybrid maltose
map had many more experimental conformations within its 2 kcal/mol
contour than did the QM F-maltose map. The apparent mean strength
of an intra-molecular, inter-residue hydrogen bond is about
3 kcal/mol, based on the energy for many of the hydrogen bonded
maltose sturctures on the F-maltose map. The F-maltose map was
similar to a new QM map for an analog of maltose in which all
hydroxyl groups were replaced with hydrogen atoms.