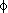 and
and
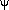 varying over the whole
360° range.
Sucrose is modeled by a THP-O-THF analog (shown below together with the
crystal structure of sucrose), the trehaloses
and cellobiose by their THP-O-THP analogs.
varying over the whole
360° range.
Sucrose is modeled by a THP-O-THF analog (shown below together with the
crystal structure of sucrose), the trehaloses
and cellobiose by their THP-O-THP analogs.
The potential energy surfaces of a number of disaccharide models were
investigated by MM3(96) and ab initio
RHF/6-31G* calculations using the two
interlinkage angles 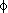 and
and
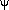 varying over the whole
360° range.
Sucrose is modeled by a THP-O-THF analog (shown below together with the
crystal structure of sucrose), the trehaloses
and cellobiose by their THP-O-THP analogs.
varying over the whole
360° range.
Sucrose is modeled by a THP-O-THF analog (shown below together with the
crystal structure of sucrose), the trehaloses
and cellobiose by their THP-O-THP analogs.
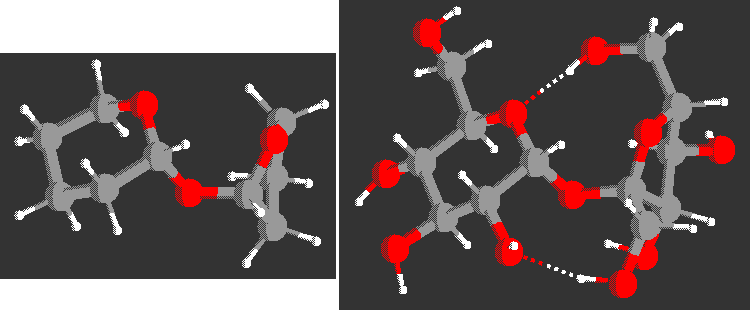
Minima and saddle points were located by MP2/6-31G* and DFT B3LYP/6-31G* methods. Comparison with the crystallographic data of di-, tri-, and tetra-saccharides show the crystal structures in the low energy regions of the analog maps. In contrast, the MM3(96) force field cannot represent the experimental results of sucrose correctly.
The discrepances in the energetic behaviour of the located minima can be explained by the overlap of anomeric and exo-anomeric effects of the two rings. Basis sets such as cc-pVDZ, aug-cc-pVDZ and 6-31+G were used to estimate the diffuse distribution of the lone pair density of the anomeric oxygens, which hyperconjugate into the anomeric bonds. CHELPG and NPA charges were used to estimate the dipole-dipole interactions of the important minima.
A Fourier analysis is employed to determine the strength of the different electronic effects (dipole-dipole interactions, anomeric effects/hyperconjugation, bond staggering effects) in the disaccharide models.