M. Ramek,
Ab-initio SCF Investigation of the Intramolecular
Hydrogen Bonding in epsilon-Aminohexanoic Acid,
Int. J. Quant. Chem.: Quant. Biol. Symp. 21, 79-93 (1994).

 -aminohexanoic acid, which contain
an intramolecular N···H-O hydrogen bond.
Comparison of characteristic data with those of the homologs
with fewer carbon atoms shows that
-aminohexanoic acid, which contain
an intramolecular N···H-O hydrogen bond.
Comparison of characteristic data with those of the homologs
with fewer carbon atoms shows that 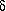 -aminopentanoic acid forms the most stable hydrogen
bonds. In contrast to these homologs, the -COOH group is not limited
to anti-periplanar orientation in the H-bonded conformers
of
-aminopentanoic acid forms the most stable hydrogen
bonds. In contrast to these homologs, the -COOH group is not limited
to anti-periplanar orientation in the H-bonded conformers
of