Intramolecular Hydrogen Bonds in Amino Aldehydes.
 |
deutsche Version. |
| © |
Copyright note. |
 |
Site-map. |
Intramolecular Hydrogen Bonds in Amino Aldehydes. |
|
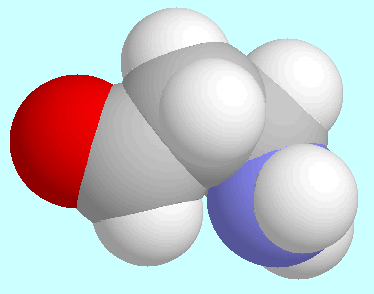 Evaluation of all intramolecular interactions in
3-aminopropanal,
Evaluation of all intramolecular interactions in
3-aminopropanal,
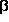 -alanine, and 3-aminopropanol
shows that the hydrogen bond
N···H-O is the strongest interaction in
all of these compounds. Consequently all conformers, in which this
interaction is present, are of considerable kinetical stability: the
lowest potential barriers are 28.66 kJ/mol
-alanine, and 3-aminopropanol
shows that the hydrogen bond
N···H-O is the strongest interaction in
all of these compounds. Consequently all conformers, in which this
interaction is present, are of considerable kinetical stability: the
lowest potential barriers are 28.66 kJ/mol ![]()
 -alanine)
-alanine)
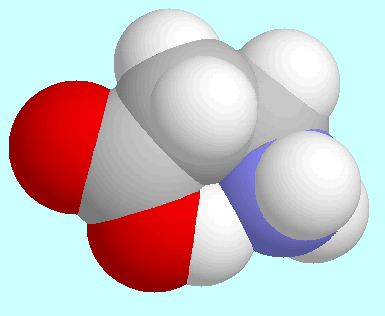 The expectation that this strongest interaction has
the biggest impact on the potential energy surface (PES)
is, however, misleading. This is already
obvious from the geometries of those conformers, in which it is
present, as discussed in the previous page of this tour.
Hence it may be concluded that the CO group rather than the hydrogen
bond N···H-O has the biggest impact on the PES of
those compounds, in which it is present. This conclusion,
which also emerges from the comparison
of the series of homologous
The expectation that this strongest interaction has
the biggest impact on the potential energy surface (PES)
is, however, misleading. This is already
obvious from the geometries of those conformers, in which it is
present, as discussed in the previous page of this tour.
Hence it may be concluded that the CO group rather than the hydrogen
bond N···H-O has the biggest impact on the PES of
those compounds, in which it is present. This conclusion,
which also emerges from the comparison
of the series of homologous  -amino acids and
-amino acids and  -amino alcohols, also explains
the similarity of the PES of 3-aminopropanal and
-amino alcohols, also explains
the similarity of the PES of 3-aminopropanal and  -alanine. This similarity is not
only obvious from the geometries and the electron densities of the
global minima, but also from the geometries of all other conformers.
For the
-alanine. This similarity is not
only obvious from the geometries and the electron densities of the
global minima, but also from the geometries of all other conformers.
For the  -alanine
conformer with the N···H-O
hydrogen bond and its 3-aminopropanal match this similarity of geometries is
impressive in view of the fact, that the electronic interaction
repulsive in the latter (causing, e.g., completely different reactions of
the two conformers upon rotation of the -CO(R) group).
-alanine
conformer with the N···H-O
hydrogen bond and its 3-aminopropanal match this similarity of geometries is
impressive in view of the fact, that the electronic interaction
repulsive in the latter (causing, e.g., completely different reactions of
the two conformers upon rotation of the -CO(R) group).
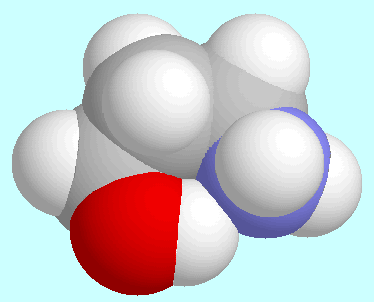 The conclusion, that the CO group influences the PES more than any
other structural feature, is also supported by the fact, that the OH
group has three stable orientations relative to the rest of the
molecule in 3-aminopropanol (or any other alcohol) but only two in
The conclusion, that the CO group influences the PES more than any
other structural feature, is also supported by the fact, that the OH
group has three stable orientations relative to the rest of the
molecule in 3-aminopropanol (or any other alcohol) but only two in
 -alanine
(or any other carbon acid) due to the strong electrostatic
interaction between the groups CO and OH.
-alanine
(or any other carbon acid) due to the strong electrostatic
interaction between the groups CO and OH.