| Intramolecular Hydrogen Bonds in Amino Acids. |
 |
deutsche Version. |
Zwitterionic versus Neutral Form.
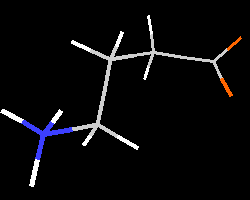 The structural behaviour of amino acids is exemplified here for
The structural behaviour of amino acids is exemplified here for
 -aminobutyric acid
(GABA). The zwitterionic form, as determined via crystal structure analysis,
was used as the initial geometry in an ab initio
geometry optimization, in which the maximum geometry change
was kept deliberately small.
Immediately, the trans
orientation of the N-C-C-C fragment starts to bend and
the -NH
-aminobutyric acid
(GABA). The zwitterionic form, as determined via crystal structure analysis,
was used as the initial geometry in an ab initio
geometry optimization, in which the maximum geometry change
was kept deliberately small.
Immediately, the trans
orientation of the N-C-C-C fragment starts to bend and
the -NH
 group approaches the -COO
group approaches the -COO group. After 33 geometry steps a
group. After 33 geometry steps a  -H···O-CO-
-H···O-CO- ···HOOC-
···HOOC-
Initial and final geometry are shown here; an animation of the complete
sequence (100K, playing time: 30s) is available on a
separate page.
Informations required by Austrian law (Offenlegung gem. §25 MedienG): Dr. Michael Ramek, Graz.