Intramolecular Hydrogen Bonds in Amino Acids.
 | deutsche Version. |
 |
Site-map. |
Intramolecular Hydrogen Bonds in Amino Acids. |
|
Amino acids,
besides being the building blocks of peptides and proteins,
are of special interest to structural chemistry. The reason of
this special interest is the fact that amino acids form zwitterions
H N
N -X-COO
-X-COO in the solid state and in polar media, but
neutral molecules
in the solid state and in polar media, but
neutral molecules  N-X-COOH
N-X-COOH![]() Zwitterion to neutral conversion,
Zwitterion to neutral conversion,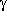 -aminobutyric
-aminobutyric
Amino acids form very stable crystals that, under atmospheric pressure, in most cases decompose before melting. Experimental studies of the neutral form therefore require sophisticated techniques. Quantum chemical calculations, in contrast, deal with isolated molecules in vacuo and require additional concepts to treat any effects of the molecular environment. Quantum chemical calculations are therefore an ideal tool for the study of neutral amino acids.
The following pages give selected results of an ab initio
study of  -amino
-amino -alanine,
-alanine, -aminobutyric
-aminobutyric