Intramolecular Hydrogen Bonds in Amino Acids.
 |
deutsche Version. |
| © |
Copyright note. |
 |
Site-map. |
Intramolecular Hydrogen Bonds in Amino Acids. |
|
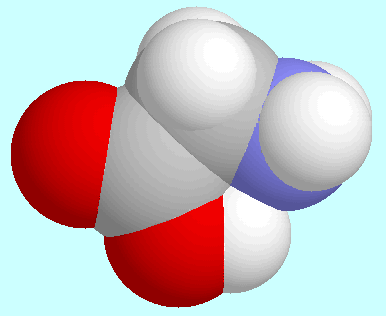 Glycine forms one conformer with a O-H···N hydrogen bond.
With split-valence basis
sets, this conformer is mirror symmetrical, whereas basis sets with
polarization functions yield a similar, but slightly unsymmetrical geometry.
Therefore, in the latter case there are strictly speaking two H-bonded
local minima in the potential energy surface, but the barrier between
these two is extremely low and vibrational averaging takes place soon.
Glycine forms one conformer with a O-H···N hydrogen bond.
With split-valence basis
sets, this conformer is mirror symmetrical, whereas basis sets with
polarization functions yield a similar, but slightly unsymmetrical geometry.
Therefore, in the latter case there are strictly speaking two H-bonded
local minima in the potential energy surface, but the barrier between
these two is extremely low and vibrational averaging takes place soon.
The H-bonded conformer is not the global minimum of the
potential energy surface, which has made history in the field
of quantum chemistry: it was first identified from the gas-phase microwave
spectrum as the most stable form, because of the high intensity of its
signals, whereas the ab initio
results indicated a mirror symmetrical conformation
without hydrogen bonds as the global minimum.
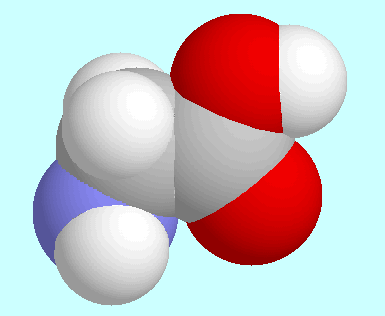 Based on the predictions for the rotational constants of this form,
this conformer could then also be
identified in the microwave spectrum. The apparant difference between
theoretical and experimental results found a simple explanation:
the higher microwave intensity of the
H-bonded form is not caused by higher population, which means lower
energy, but by the significantly higher dipole moment that is due
to the hydrogen bond.
Since this episode, glycine has not only
been the topic of several investigations, but also served as
a test molecule for new quantum chemical techniques.
Based on the predictions for the rotational constants of this form,
this conformer could then also be
identified in the microwave spectrum. The apparant difference between
theoretical and experimental results found a simple explanation:
the higher microwave intensity of the
H-bonded form is not caused by higher population, which means lower
energy, but by the significantly higher dipole moment that is due
to the hydrogen bond.
Since this episode, glycine has not only
been the topic of several investigations, but also served as
a test molecule for new quantum chemical techniques.
 -alanine.
-alanine.