Intramolecular Hydrogen Bonds in Amino Acids.
 |
deutsche Version. |
| © |
Copyright note. |
 |
Site-map. |
Intramolecular Hydrogen Bonds in Amino Acids. |
|
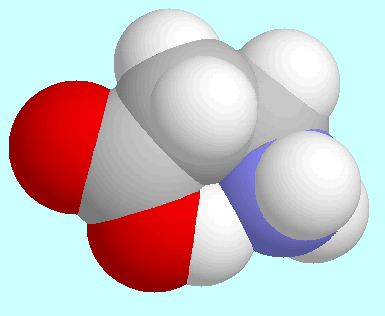
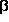 -alanine
forms one symmetry unique conformer with a
O-H···N hydrogen bond. This
conformer has a non-symmetric geometry that is of the
envelope-type: the atoms of the COOH group,
the
-alanine
forms one symmetry unique conformer with a
O-H···N hydrogen bond. This
conformer has a non-symmetric geometry that is of the
envelope-type: the atoms of the COOH group,
the  carbon
carbon carbon atom
sticks out of this plane.
Due to this non-symmetry, there are in fact two H-bonded conformers
in the ab initio
potential energy surface, namely the one shown in the display and
its mirror image.
carbon atom
sticks out of this plane.
Due to this non-symmetry, there are in fact two H-bonded conformers
in the ab initio
potential energy surface, namely the one shown in the display and
its mirror image.
 Although they do not have any asymmetric carbon
atoms, the two H-bonded conformers thus constitute an
enantiomer pair, since
they are unsymmetric and mirror images of each other. This of special
interest, because there exists a H-bond preserving reaction that
interconnects these two conformers without breaking the hydrogen bond.
The transition state of this reaction path is not the mirror
symmetrical conformation, in which the cycle H-O-C-C-C-N forms a planar
(but irregular) hexagon. Instead, there are two non-symmetrical
transition states, in which
the amino group is twisted approximately 20°off the symmetrical
orientation (clockwise or counter-clockwise).
Hence there are two (energetically equivalent)
H-bond preserving reaction paths that connect the
two enantiomers, both of which avoid the point of achirality and thus remain
chiral (although changing parity) from beginning to end.
The corresponding segment of the energy hypersurface in the vicinity
of this point of achirality is shown in the graphic next to this paragraph.
Although they do not have any asymmetric carbon
atoms, the two H-bonded conformers thus constitute an
enantiomer pair, since
they are unsymmetric and mirror images of each other. This of special
interest, because there exists a H-bond preserving reaction that
interconnects these two conformers without breaking the hydrogen bond.
The transition state of this reaction path is not the mirror
symmetrical conformation, in which the cycle H-O-C-C-C-N forms a planar
(but irregular) hexagon. Instead, there are two non-symmetrical
transition states, in which
the amino group is twisted approximately 20°off the symmetrical
orientation (clockwise or counter-clockwise).
Hence there are two (energetically equivalent)
H-bond preserving reaction paths that connect the
two enantiomers, both of which avoid the point of achirality and thus remain
chiral (although changing parity) from beginning to end.
The corresponding segment of the energy hypersurface in the vicinity
of this point of achirality is shown in the graphic next to this paragraph.
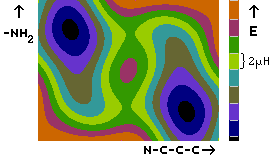
 As in glycine,
the H-bonded conformers are not the global minima of the
potential energy surface. The reason for this is that the COOH
group is about 35 kJ/mol more stable in the cis orientation, where
the groups C=O and O-H point to the same side of the C-O bond, than
in the trans orientation that is necessary to form the H-bond.
Thus, the energy gain by the hydrogen bond formation (about 32 kJ/mol)
is cancelled by the energy loss of the reorientation of the COOH group.
The geometry of the global minima is characterized by the COOH group,
the
As in glycine,
the H-bonded conformers are not the global minima of the
potential energy surface. The reason for this is that the COOH
group is about 35 kJ/mol more stable in the cis orientation, where
the groups C=O and O-H point to the same side of the C-O bond, than
in the trans orientation that is necessary to form the H-bond.
Thus, the energy gain by the hydrogen bond formation (about 32 kJ/mol)
is cancelled by the energy loss of the reorientation of the COOH group.
The geometry of the global minima is characterized by the COOH group,
the  , and the
, and the
 carbon atom in one plane, and the nitrogen
atom sticking out of that plane. The carbonyl oxygen atom and one
amino group hydrogen atom come close to each other in this orientation,
but not close enough to form a hydrogen bond.
carbon atom in one plane, and the nitrogen
atom sticking out of that plane. The carbonyl oxygen atom and one
amino group hydrogen atom come close to each other in this orientation,
but not close enough to form a hydrogen bond.
 -aminobutyric acid.
-aminobutyric acid.