Intramolecular Hydrogen Bonds in Amino Acids.
 |
deutsche Version. |
| © |
Copyright note. |
 |
Site-map. |
Intramolecular Hydrogen Bonds in Amino Acids. |
|
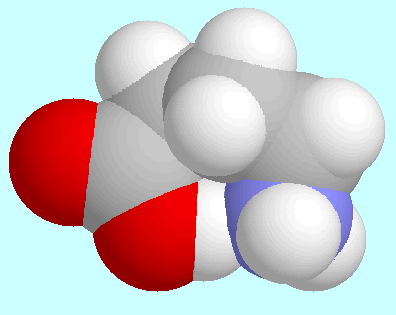
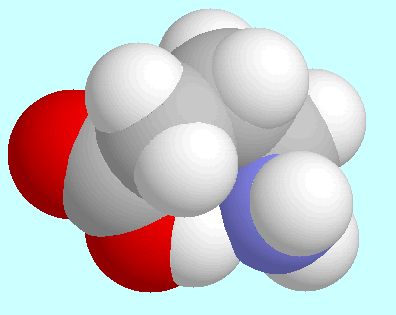
 -aminobutyric acid
is physiologically important as an inhibitory neurotransmitter
substance, and thus has been given
a commonly used abbreviation: GABA. The neutral form of GABA
forms a total of four conformers with O-H···N
hydrogen bonds,
which form two pairs of enantiomers.
One of these enantiomer pairs exhibits an envelope structure
with the COOH group, the nitrogen atom, and the carbon atoms
-aminobutyric acid
is physiologically important as an inhibitory neurotransmitter
substance, and thus has been given
a commonly used abbreviation: GABA. The neutral form of GABA
forms a total of four conformers with O-H···N
hydrogen bonds,
which form two pairs of enantiomers.
One of these enantiomer pairs exhibits an envelope structure
with the COOH group, the nitrogen atom, and the carbon atoms  and
and  in one (slightly distorted) plane,
whereas the other has carbon atoms
in one (slightly distorted) plane,
whereas the other has carbon atoms
 and
and 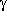 out
of the plane of the COOH group,
carbon atom
out
of the plane of the COOH group,
carbon atom  ,
and the nitrogen atom.
The hydrogen bonds are of different strength in these two pairs:
stronger in the former, and weaker in the latter. The overlap of
the fused spheres in the displays show this different strength
clearly by the different amount of overlap.
,
and the nitrogen atom.
The hydrogen bonds are of different strength in these two pairs:
stronger in the former, and weaker in the latter. The overlap of
the fused spheres in the displays show this different strength
clearly by the different amount of overlap.
 As in glycine and
As in glycine and  -alanine, the H-bonded conformers are not the global minima of the
ab initio
potential energy surface. The conformation of the global minima of GABA
is a straightforward extension of those of
-alanine, the H-bonded conformers are not the global minima of the
ab initio
potential energy surface. The conformation of the global minima of GABA
is a straightforward extension of those of  -alanine: the COOH group
and the carbon atoms
-alanine: the COOH group
and the carbon atoms  and
and
 are located in one plane, carbon
atom
are located in one plane, carbon
atom  sticks out of that plane, and the amino group is oriented
towards the COOH group. Again, the interaction between the carbonyl
oxygen atom and the amino group hydrogen atom does not qualify as a
hydrogen bond.
sticks out of that plane, and the amino group is oriented
towards the COOH group. Again, the interaction between the carbonyl
oxygen atom and the amino group hydrogen atom does not qualify as a
hydrogen bond.
 In fact, among the 62 symmetry-unique local
minima in the potential energy surface of GABA there is only one,
in which a N-H···O=C hydrogen bond is formed; the geometry
of this high-energetical conformer, which has the COOH-group in the
unfavourable trans-orientation, is shown next to this paragraph.
It should be noted, that this conformer and its enantiomer,
together with the conformers with the strong
N···H-O bond, are the only
hydrogen bonded conformers in the GABA potential energy surface.
In fact, among the 62 symmetry-unique local
minima in the potential energy surface of GABA there is only one,
in which a N-H···O=C hydrogen bond is formed; the geometry
of this high-energetical conformer, which has the COOH-group in the
unfavourable trans-orientation, is shown next to this paragraph.
It should be noted, that this conformer and its enantiomer,
together with the conformers with the strong
N···H-O bond, are the only
hydrogen bonded conformers in the GABA potential energy surface.
As in  -alanine,
there are reaction paths that preserve the
N···H-O
hydrogen bond.
In the case of GABA, there are two energetically different paths that
connect the different H-bonded conformers in a closed cycle
A-B-Am-Bm-A ("m" denoting mirror images).
Each H-bonded conformer therefore
has the "choice" between two different paths that do not break
-alanine,
there are reaction paths that preserve the
N···H-O
hydrogen bond.
In the case of GABA, there are two energetically different paths that
connect the different H-bonded conformers in a closed cycle
A-B-Am-Bm-A ("m" denoting mirror images).
Each H-bonded conformer therefore
has the "choice" between two different paths that do not break
![]() the hydrogen
bond. In contrast to 3-aminoproanol,
where there is a similar cycle of hydrogen bond conserving reactions,
the hydrogen
bond. In contrast to 3-aminoproanol,
where there is a similar cycle of hydrogen bond conserving reactions,
![]() the
reactions of the GABA cycle are the ones with the lowest potential barriers for
the
reactions of the GABA cycle are the ones with the lowest potential barriers for
![]() both H-bonded
conformers.
both H-bonded
conformers.
 -alanine.
-alanine.
 -aminopentanoic acid.
-aminopentanoic acid.