Structure Determination of Auxin Phytohormones.
 |
deutsche Version.
|
| © |
Copyright note. |
 |
Site-map. |
Structure Determination of Auxin Phytohormones. |
|
The results for various derivatives of 3-indole acetic acid (IAA) show that the shape of the potential energy surface (PES) is strongly influenced by the substituent at position 4. In particular, the sequence 4-Cl-IAA, 4-Me-IAA, and IAA is remarkable: the PES of IAA and 4-Cl-IAA are quite different, and that of 4-Me-IAA is somewhere inbetween these two, showing elements of both extremes. Therefore the idea was tempting to extend this series in a suitable manner. Since the combined steric and electronic effect of the substituent in 4-Cl-IAA is very effective in blocking the side chain, the other end of the series seemed to be more promising: changing the C-H fragment into a nitrogen atom should reduce the steric impact to a minimum.
| T1 | T2 | E [kJ/mol] |
|---|---|---|
| 0.00° | 0.00° | 0.000 |
| 100.85° | -15.68° | 5.611 |
| 47.71° | 115.07° | 8.112 |
| 114.84° | 145.56° | 8.648 |
1-H-Pyrrolo[3,2-b]pyridine-3-yl acetic acid (PPAA) is this constructed extension of the series 4-Cl-IAA, 4-Me-IAA, IAA, the planar forms of which are shown on top of this paragraph. The potential energy surface of PPAA contains four symmetry-unique local minima with cis-orientation of the COOH group, K, L, M, and N, the 6-31G* optimized data of which are listed in the table. The geometries of these conformers correspond well with those of the 4-Cl-IAA energy minima, except that M is shifted to a higher T1-value because of an attractive C-H···N interaction (H···N distance: 2.736 Å). The relative energies differ, however: B and C are more stable in the 4-Cl-PES than their counterparts L and N, whereas the kinetically instable D of 4-Cl-IAA corresponds to M, which is stabilized by the attractive intramolecular interaction mentioned above.
These minima are interconnected among themselves and their mirror images l, m, and n by a network of reaction paths, which looks very similar to that of 4-Cl-IAA. The major difference is the conformation with T1=0° and T2=180°, which is a saddle point (D-d) in the 4-Cl case but a second order saddle point in the PES of PPAA. The geometries of these two conformations, which are shown next to this paragraph, indicate a combination of electronic and steric effects for this difference: in 4-Cl-IAA the torsion of the acetic acid side chain is sterically hindered, whereas in PPAA it leads to an improved N···H-C interaction.
PPAA also forms a conformer,
in which the trans-oriented COOH group is engaged in
an intramolecular hydrogen bond. Two views of this conformer are shown
next to this paragraph. In the PPAA case, the H-bonded conformer is of
considerable stability: it has a
relative energy (RHF/6-31G*) of 5.802 kJ/mol, a potential
barrier of 42.505 kJ/mol in the reaction to N, and a
hydrogen bond (N···H distance 2.020 Å,
bond order 0.069) that is comparable in strength to that in
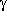 -aminobutyric acid, which also leads to a
seven-membered ring.
-aminobutyric acid, which also leads to a
seven-membered ring.