Michael Ramek,
Ching-Hsing Yu, Joshua Sakon, and Lothar Schäfer,
Ab Initio Study of the Conformational Dependence of the
Nonplanarity of the Peptide Group
J. Phys. Chem. A, 104, 9636-9645 (2000).

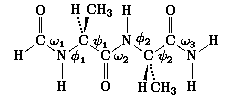

 , of the central
peptide group in N-formyl-L-alanyl-L-alanine amide (ALA-ALA) was
investigated using
a database of 11 664 RHF/4-21G
, of the central
peptide group in N-formyl-L-alanyl-L-alanine amide (ALA-ALA) was
investigated using
a database of 11 664 RHF/4-21G 
 ,
,