M. Ramek and P. I. Nagy,
Theoretical Investigation of the Neutral / Zwitterionic Equilibrium
of gamma-Aminobutyric Acid (GABA) Conformers in Aqueous Solution,
J. Phys. Chem. A 104, 6844-6854 (2000).

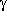 -aminobutyric acid does not form a stable zwitterionic species
in the gas phase, as calculated at the HF/6-311++G** level, the GABA·2H2O
system was optimized for several neutral and zwitterionic GABA
tautomers/conformers. The obtained molecular geometries and vibrational
frequencies determined for the dihydrates reflect structural changes
for GABA due to close and strongly bound water molecules. By use of
GABA geometries optimized in the dihydrates, relative free energies of
different species in aqueous solution were calculated.
MP2/6-311++G**//HF/6-311++G** energy values show that the
neutral form is strongly preferred over the zwitterionic one for the
isolated GABA species. The neutral tautomer, which is most stable in the
gas phase, is only marginally changed by hydration; it is without an
intramolecular hydrogen bond and has nearly gauche-gauche arrangements,
54° and -83°, for the NCCC and CCCC torsion angles, respectively,
as determined in the dihydrate. In aqueous solution the zwitterionic
structure is dominant. Comparison of cyclic gauche-gauche forms and a partially
extended gauche-trans structure indicates the preference for the more
extended form. This structure differs from the trans-gauche zwitterionic
conformer found for GABA by X-ray experiments in the crystalline phase.
The experimental GABA conformer is not stable either in the isolated form
or in the gas-phase dihydrate. It is, however, more stable by about
6.5 kcal/mole than the gas-phase gauche-trans form, as turned out in a
restricted geometry optimization. Such a large internal stabilization
may allow the existence (even preference) of the experimental zwitterionic
GABA structure in aqueous solution if solvent effects are preferable.
Partition of GABA between water and chloroform is not favoured.
At least 7.5 kcal/mole free energy increase is required if the
zwitterion either directly or after transformation to a neutral form
would leave the aqueous phase and enter chloroform. This result supports
the experimental finding that GABA does not cross the blood-brain barrier.
-aminobutyric acid does not form a stable zwitterionic species
in the gas phase, as calculated at the HF/6-311++G** level, the GABA·2H2O
system was optimized for several neutral and zwitterionic GABA
tautomers/conformers. The obtained molecular geometries and vibrational
frequencies determined for the dihydrates reflect structural changes
for GABA due to close and strongly bound water molecules. By use of
GABA geometries optimized in the dihydrates, relative free energies of
different species in aqueous solution were calculated.
MP2/6-311++G**//HF/6-311++G** energy values show that the
neutral form is strongly preferred over the zwitterionic one for the
isolated GABA species. The neutral tautomer, which is most stable in the
gas phase, is only marginally changed by hydration; it is without an
intramolecular hydrogen bond and has nearly gauche-gauche arrangements,
54° and -83°, for the NCCC and CCCC torsion angles, respectively,
as determined in the dihydrate. In aqueous solution the zwitterionic
structure is dominant. Comparison of cyclic gauche-gauche forms and a partially
extended gauche-trans structure indicates the preference for the more
extended form. This structure differs from the trans-gauche zwitterionic
conformer found for GABA by X-ray experiments in the crystalline phase.
The experimental GABA conformer is not stable either in the isolated form
or in the gas-phase dihydrate. It is, however, more stable by about
6.5 kcal/mole than the gas-phase gauche-trans form, as turned out in a
restricted geometry optimization. Such a large internal stabilization
may allow the existence (even preference) of the experimental zwitterionic
GABA structure in aqueous solution if solvent effects are preferable.
Partition of GABA between water and chloroform is not favoured.
At least 7.5 kcal/mole free energy increase is required if the
zwitterion either directly or after transformation to a neutral form
would leave the aqueous phase and enter chloroform. This result supports
the experimental finding that GABA does not cross the blood-brain barrier.