Intramolecular Hydrogen Bonds in Amino Acids.
 |
deutsche Version. |
| © |
Copyright note. |
 |
Site-map. |
Intramolecular Hydrogen Bonds in Amino Acids. |
|
 All
All  -amino acids
are capable of forming
a N···H-O hydrogen bond between the two terminal groups
-COOH and -NH
-amino acids
are capable of forming
a N···H-O hydrogen bond between the two terminal groups
-COOH and -NH . Up to
. Up to  -aminopentanoic acid,
this hydrogen bond can only be formed if the COOH group is in
trans-orientation, which is energetically unfavourable.
For this reason none of the hydrogen bonded conformers is the
global minimum of the ab initio energy surface.
-aminopentanoic acid,
this hydrogen bond can only be formed if the COOH group is in
trans-orientation, which is energetically unfavourable.
For this reason none of the hydrogen bonded conformers is the
global minimum of the ab initio energy surface.
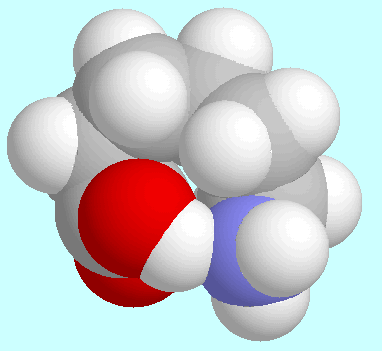 The strength of the hydrogen bonds shows a steady increase
up to
The strength of the hydrogen bonds shows a steady increase
up to 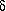 -aminopentanoic acid, in
which the hydrogen bond closes an
eight-membered ring. In
-aminopentanoic acid, in
which the hydrogen bond closes an
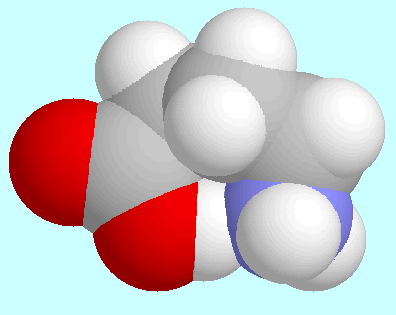
eight-membered ring. In  -aminohexanoic acid the hydrogen bond
is weaker again, due to steric hinderings, but several hydrogen
bonded conformers exist in which the COOH group is in a distorted
cis-orientation. It can be anticipated that
zeta-aminoheptanoic acid will allow a conformation with a
ten-membered ring that is closed by a hydrogen bond, in which the COOH group
is in a pure, undistorted cis-orientation.
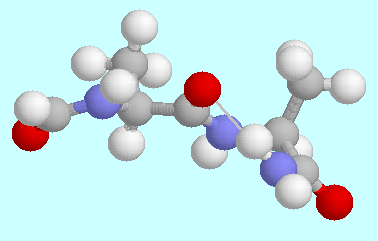
-aminohexanoic acid the hydrogen bond
is weaker again, due to steric hinderings, but several hydrogen
bonded conformers exist in which the COOH group is in a distorted
cis-orientation. It can be anticipated that
zeta-aminoheptanoic acid will allow a conformation with a
ten-membered ring that is closed by a hydrogen bond, in which the COOH group
is in a pure, undistorted cis-orientation.
 The finding that it requires a
ten-membered ring
to close a hydrogen
bond, without the disadvantage of unfavourable orientations in
another part of the molecule, gives a deep insight into peptide and
protein chemistry. Peptides and proteins are built up by
The finding that it requires a
ten-membered ring
to close a hydrogen
bond, without the disadvantage of unfavourable orientations in
another part of the molecule, gives a deep insight into peptide and
protein chemistry. Peptides and proteins are built up by
 -amino acids
and are able to
form intramolecular C=O···H-N bonds that are similar to
the hydrogen bonds in
-amino acids
and are able to
form intramolecular C=O···H-N bonds that are similar to
the hydrogen bonds in  -amino acids.
These intramolecular hydrogen bonds are responsible for the over-all structure,
and thus to the function of proteins and peptides. Due to the
building principle of the peptide chain, the size of the rings, which are
formed by these H-bonds, is limited to a few different values:
-amino acids.
These intramolecular hydrogen bonds are responsible for the over-all structure,
and thus to the function of proteins and peptides. Due to the
building principle of the peptide chain, the size of the rings, which are
formed by these H-bonds, is limited to a few different values:
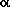 -helices
are stabilized by hydrogen bonds that close 13-membered rings;
there are two different
-helices
are stabilized by hydrogen bonds that close 13-membered rings;
there are two different  -helices: right-handed and left-handed.
-helices: right-handed and left-handed.
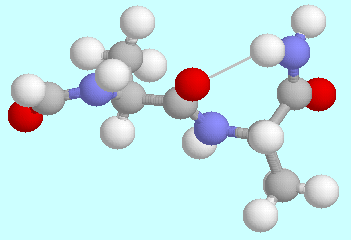
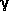 -turns, which
create a kink in the peptide chain, contain a seven-membered
ring;
-turns, which
create a kink in the peptide chain, contain a seven-membered
ring; 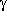 -turns also
occur in two different forms,
up and down, which are shown in the
graphical inserts next to this and the previous paragraph, using the
model tripeptide
HCO-L-Ala-L-Ala-NH
-turns also
occur in two different forms,
up and down, which are shown in the
graphical inserts next to this and the previous paragraph, using the
model tripeptide
HCO-L-Ala-L-Ala-NH as an example (the hydrogen bond is indicated as a thin line in both cases).
as an example (the hydrogen bond is indicated as a thin line in both cases).
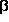 -turns, in contrast, are formed by
ten-membered hydrogen bonded rings, which, according to
the results obtained for
-turns, in contrast, are formed by
ten-membered hydrogen bonded rings, which, according to
the results obtained for  -amino
acids, allow optimal intramolecular H-bonds.
And, in fact, there are at least ten different
-amino
acids, allow optimal intramolecular H-bonds.
And, in fact, there are at least ten different
 -turns, which are
responsible for a number of different secondary structure elements,
such as the hair-pin motif (stick model)
or the 3
-turns, which are
responsible for a number of different secondary structure elements,
such as the hair-pin motif (stick model)
or the 3
 -helix.
-helix.  -aminohexanoic acid.
-aminohexanoic acid.