Intramolecular Hydrogen Bonds in Hydroxy Acids.
 |
deutsche Version. |
| © |
Copyright note. |
 |
Site-map. |
Intramolecular Hydrogen Bonds in Hydroxy Acids. |
|
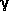 -hydroxybutyric acid
is one of the few substances, which are able to pass the barrier between
blood and brain. This physiological property has earned
-hydroxybutyric acid
is one of the few substances, which are able to pass the barrier between
blood and brain. This physiological property has earned
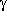 -hydroxybutyric acid
the abbreviation "GHB". It occurs naturally in the mammelian brain, where it is
synthesized and released
by specific neuronal circuits, which possess neuromodulatory functions.
The synthesis in the brain involves
-hydroxybutyric acid
the abbreviation "GHB". It occurs naturally in the mammelian brain, where it is
synthesized and released
by specific neuronal circuits, which possess neuromodulatory functions.
The synthesis in the brain involves 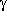 -aminobutyric acid (GABA) or 1,4-butanediol as
precursor substances. GHB can also be synthesized in the
mammelian liver from
-aminobutyric acid (GABA) or 1,4-butanediol as
precursor substances. GHB can also be synthesized in the
mammelian liver from 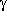 -butyrolactone.
GHB is able to substitute ethanol during ethanol withdrawal and
has been used in the treatment of alcohol dependence as well as
narcolepsy.
In the recent years, however, it has also been used as an ingredient of party
drugs.
-butyrolactone.
GHB is able to substitute ethanol during ethanol withdrawal and
has been used in the treatment of alcohol dependence as well as
narcolepsy.
In the recent years, however, it has also been used as an ingredient of party
drugs.
|
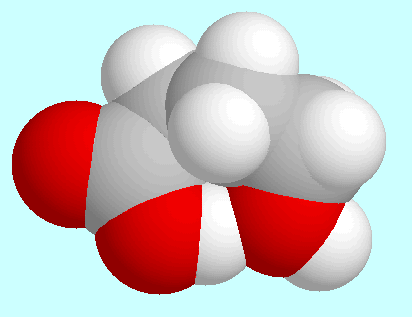
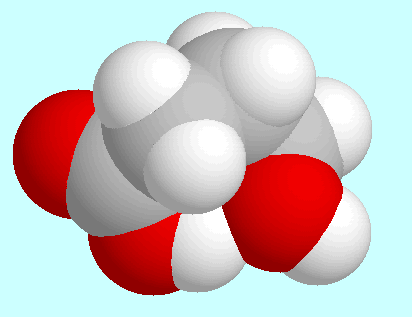
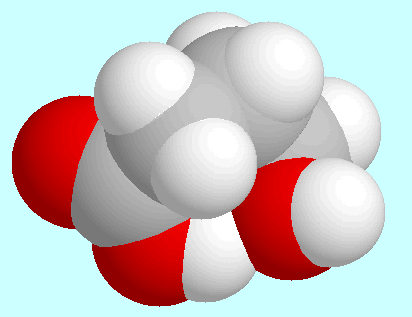
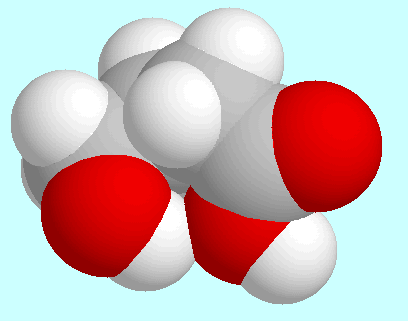
Regarding intramolecular interactions, three symmetry-unique conformers
have to be named in first place, all of which form a
hydrogen bond (CO)O-H···O-H. These
three conformers are shown on top of
this paragraph in the order of their energies. The most stable (A)
is of the envelope-type: the COOH-group, the carbon atoms
 and
and 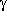 , and the aliphatic OH-group
are located approximately in one common plane, whereas the carbon atom
, and the aliphatic OH-group
are located approximately in one common plane, whereas the carbon atom
 sticks out of that plane. The two other conformers (B and C) have a kind of
distorted boat form and differ only in the orientation of the OH-group.
The hydrogen bond in these two is weaker than in A, which can already
be seen from the overlap in the fused sphere models.
A network of hydrogen bond preserving reactions exists
between these three conformers and their enantiomers (a, b, c), which
is shown in the following scheme.
sticks out of that plane. The two other conformers (B and C) have a kind of
distorted boat form and differ only in the orientation of the OH-group.
The hydrogen bond in these two is weaker than in A, which can already
be seen from the overlap in the fused sphere models.
A network of hydrogen bond preserving reactions exists
between these three conformers and their enantiomers (a, b, c), which
is shown in the following scheme.
 As in the other hydroxy and amino acids, none of these H-bonded conformers
is the global minimum of the potential energy surface due to the
energetically unfavourable trans-orientation of the COOH-group.
A numerical estimate for GHB gives a cis-trans energy difference
of 35.3 kJ/mol and H-bond stabilization energies of
34.9, 23.9, and 23.5 kJ/mol, respectively.
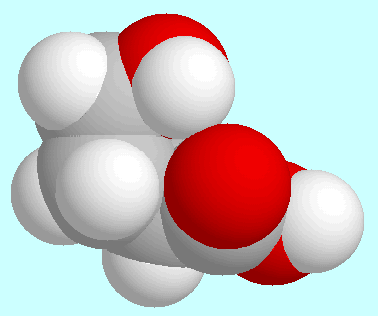
The global minimum is shown at the beginning of this paragraph. In it,
the COOH-group is almost coplanar with the carbon atoms
As in the other hydroxy and amino acids, none of these H-bonded conformers
is the global minimum of the potential energy surface due to the
energetically unfavourable trans-orientation of the COOH-group.
A numerical estimate for GHB gives a cis-trans energy difference
of 35.3 kJ/mol and H-bond stabilization energies of
34.9, 23.9, and 23.5 kJ/mol, respectively.
The global minimum is shown at the beginning of this paragraph. In it,
the COOH-group is almost coplanar with the carbon atoms  and
and  ,
carbon atom
,
carbon atom 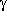 is located out of that plane, and the aliphatic OH-group
is oriented towards the carbonyl oxygen atom.
The H···O-distance in
the resulting O-H···O=C-interaction is slightly
longer than in the corresponding conformer of
is located out of that plane, and the aliphatic OH-group
is oriented towards the carbonyl oxygen atom.
The H···O-distance in
the resulting O-H···O=C-interaction is slightly
longer than in the corresponding conformer of  -hydroxypropionic acid, namely 2.33 Å.
According to the electron density between the groups OH and CO, this
interaction is not a hydrogen bond but a electrostatic attraction.
-hydroxypropionic acid, namely 2.33 Å.
According to the electron density between the groups OH and CO, this
interaction is not a hydrogen bond but a electrostatic attraction.
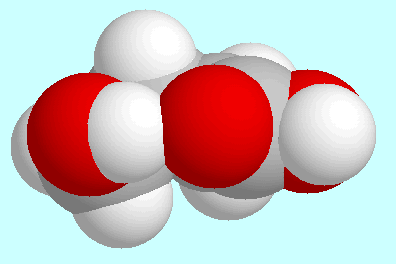
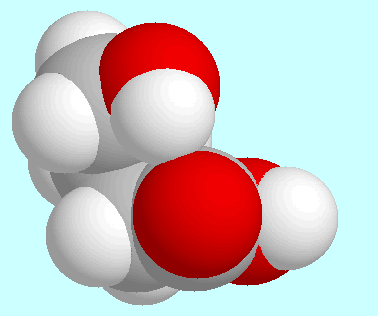 In contrast, the same interaction
O-H···O=C forms a hydrogen bond
in four other symmetry-unique conformers, namely
the two shown next to this paragraph and their analoga with
trans-orientation of the COOH-group. This hydrogen bond is weak,
however, with bond orders between 0.025 and 0.030.
In contrast, the same interaction
O-H···O=C forms a hydrogen bond
in four other symmetry-unique conformers, namely
the two shown next to this paragraph and their analoga with
trans-orientation of the COOH-group. This hydrogen bond is weak,
however, with bond orders between 0.025 and 0.030.
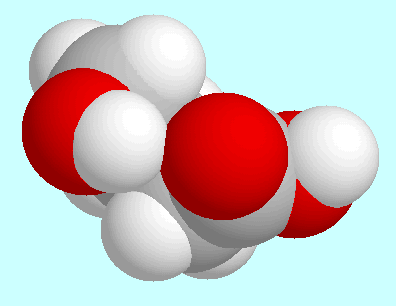
 A stronger form of this O-H···O=C
hydrogen bond (with a bond order of 0.044)
is formed in another conformer, which is shown next to this paragraph.
The geometry of this conformer can be described as a distorted envelope-type
with carbon atom
A stronger form of this O-H···O=C
hydrogen bond (with a bond order of 0.044)
is formed in another conformer, which is shown next to this paragraph.
The geometry of this conformer can be described as a distorted envelope-type
with carbon atom 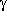 out of the plane. In this case, an energetical effect of the hydrogen bond
cannot be ruled out, since this conformer is third in energy
(out of a total of 66 symmetry-unique conformers
in the quantum chemical ab initio potential energy surface).
out of the plane. In this case, an energetical effect of the hydrogen bond
cannot be ruled out, since this conformer is third in energy
(out of a total of 66 symmetry-unique conformers
in the quantum chemical ab initio potential energy surface).
Furthermore, three conformers with a cis-orientation of the COOH-group have to be mentioned, in which a weak hydrogen bond O-H···(CO)O-H is formed. These three conformers are shown at the end of this paragraph in the order of their energy. The one shown in the center also has a high-energy analog with trans-orientation of the COOH-group.
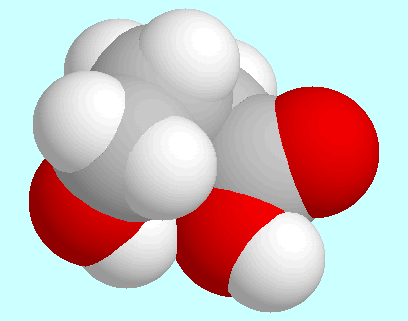
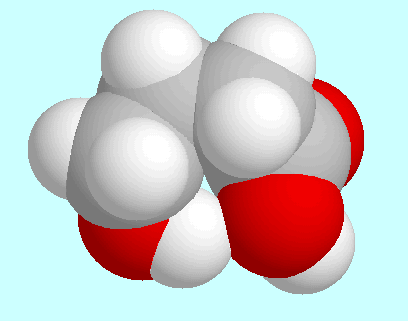
|
 -hydroxypropionic acid.
-hydroxypropionic acid.
 -hydroxypentanoic acid.
-hydroxypentanoic acid.