Intramolecular Hydrogen Bonds in Hydroxy Acids.
 |
deutsche Version. |
| © |
Copyright note. |
 |
Site-map. |
Intramolecular Hydrogen Bonds in Hydroxy Acids. |
|
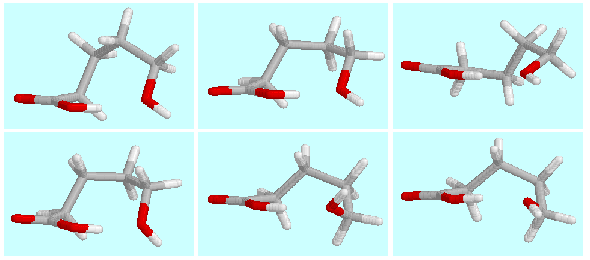
The potential energy surface of  -hydroxypentanoic acid contains six symmetry-unique local
minima with a hydrogen bond (CO)O-H···O-H.
The geometries of these minima (A, B, ... F)
are shown on top of this paragraph in the order of their energies.
They are connected among themselves and their mirror images (a, b, ..., f)
by a network of hydrogen bond preserving reactions,
as shown in the following scheme.
-hydroxypentanoic acid contains six symmetry-unique local
minima with a hydrogen bond (CO)O-H···O-H.
The geometries of these minima (A, B, ... F)
are shown on top of this paragraph in the order of their energies.
They are connected among themselves and their mirror images (a, b, ..., f)
by a network of hydrogen bond preserving reactions,
as shown in the following scheme.
Two of these reactions deserve
special attention. The first of these two was found to have a barrier
of only 0.16 kJ/mol for the conversion of D to B.
These two minima differ essentially in the orientation of the
aliphatic OH-group and have an energy difference of 1.23 kJ/mol.
In D, the unscaled harmonic vibration frequency related
to the internal rotation of the OH-group (211.24 cm )
equals a vibrational zero-point energy of 1.27 kJ/mol. This
clearly exceeds the rotational barrier, hence D is no
stable conformer although it is a true local minimum of the
potential energy surface.
A similar situation exists for E and F, which also differ in the
orientation of the OH-group only.
F has a barrier of 1.03 kJ/mol in the reaction to E, but a
vibrational zero-point energy of 1.77 kJ/mol for the
vibration frequency associated with this reaction (295.54 cm
)
equals a vibrational zero-point energy of 1.27 kJ/mol. This
clearly exceeds the rotational barrier, hence D is no
stable conformer although it is a true local minimum of the
potential energy surface.
A similar situation exists for E and F, which also differ in the
orientation of the OH-group only.
F has a barrier of 1.03 kJ/mol in the reaction to E, but a
vibrational zero-point energy of 1.77 kJ/mol for the
vibration frequency associated with this reaction (295.54 cm ).
Thus the same conclusion can be drawn for F.
).
Thus the same conclusion can be drawn for F.
In contrast to these instabilities, conformer A is of considerable stability: this can not only be seen from the large energy gap between A and B (11.62 kJ/mol), but also from the potential barriers for H-bond breaking reactions (39.5-50.1 kJ/mol) as well as for the hydrogen bond preserving ones (28.4-44.5 kJ/mol).
These differences in energetic stability are astonishing in view of the fact
that all six energy minima give a very consistent picture in terms of bond
distances: the interatomic
O···H distance is between 65% and 68% of the sum of
the van der Waals radii in all cases, and the H-bond formation leads
to an elongation of the acidic O-H and the aliphatic C-O bond
of approximately 0.011-0.013 Å (1.2-1.3%) and 0.007-0.012 Å (0.5-0.8%), respectively.
Similar to 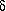 -aminopentanoic
-aminopentanoic
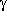 -hydroxybutyric acid.
-hydroxybutyric acid.