Intramolecular Hydrogen Bonds in Hydroxy Acids.
 |
deutsche Version. |
| © |
Copyright note. |
 |
Site-map. |
Intramolecular Hydrogen Bonds in Hydroxy Acids. |
|
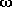 -hydroxy acids and
-hydroxy acids and
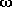 -amino acids
both form intramolecular hydrogen bonds that involve the carboxylic OH-group.
These O···H-O or
N···H-O bonds are stronger than any other intramolecular
interactions in these species. The direct comparison, which is given
below by means of two of the commonly employed criteria for hydrogen bonds,
shows that this bond is stronger in
-amino acids
both form intramolecular hydrogen bonds that involve the carboxylic OH-group.
These O···H-O or
N···H-O bonds are stronger than any other intramolecular
interactions in these species. The direct comparison, which is given
below by means of two of the commonly employed criteria for hydrogen bonds,
shows that this bond is stronger in  -amino acids, although
it is shorter in
-amino acids, although
it is shorter in  -hydroxy acids.
The latter is an effect of the
higher nuclear charge of oxygen, which also increases the
electrostatic portion of this interaction. In terms of energy,
weaker hydrogen bond and stronger electrostatic interaction on the one hand,
and stronger hydrogen bond but lesser electrostatic interaction on the other
hand add up to approximately the same stabilization energy.
Up to the seven-membered ring, which is formed in the
C4-compound, the H-bond data run more or less parallel; in the
eight-membered ring of
-hydroxy acids.
The latter is an effect of the
higher nuclear charge of oxygen, which also increases the
electrostatic portion of this interaction. In terms of energy,
weaker hydrogen bond and stronger electrostatic interaction on the one hand,
and stronger hydrogen bond but lesser electrostatic interaction on the other
hand add up to approximately the same stabilization energy.
Up to the seven-membered ring, which is formed in the
C4-compound, the H-bond data run more or less parallel; in the
eight-membered ring of 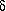 -hydroxy-
or
-hydroxy-
or 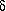 -aminopentanoic acid,
the usual criteria for hydrogen bond strength do no longer agree.
-aminopentanoic acid,
the usual criteria for hydrogen bond strength do no longer agree.
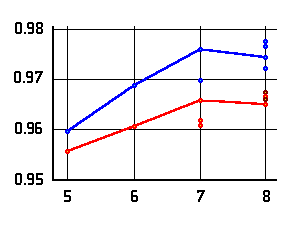
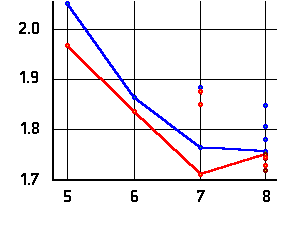
O-H distances of the acidic OH-group
in N···H-O or
O···H-O hydrogen bonded
 -amino and
-amino and
 -hydroxy acids
(left or top) and hydrogen bond distance (right or bottom)
as a function of the ring-size. Values are given in Ångströms;
amino acid data are marked in blue, hydroxy acid data in red.
The lines connect data for conformers of lowest energy.
-hydroxy acids
(left or top) and hydrogen bond distance (right or bottom)
as a function of the ring-size. Values are given in Ångströms;
amino acid data are marked in blue, hydroxy acid data in red.
The lines connect data for conformers of lowest energy.
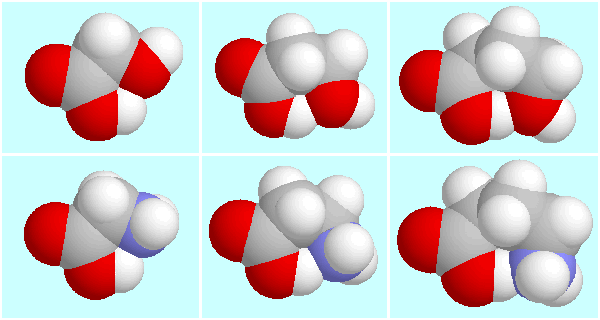
This almost parallel behaviour of the
O···H-O and N···H-O hydrogen bonds
is also present in the geometries, as
the most stable H-bonded conformer pairs of glycolic acid / glycine,
 -hydroxypropionic
acid /
-hydroxypropionic
acid /  -alanine,
and GHB / GABA show, which are displayed below.
-alanine,
and GHB / GABA show, which are displayed below.
|
While the N···H-O hydrogen bond is
considerably stronger in the  -amino
acids than its O···H-O counterpart in
the
-amino
acids than its O···H-O counterpart in
the  -hydroxy
acids, the opposite is true for all other intramolecular hydrogen bonds.
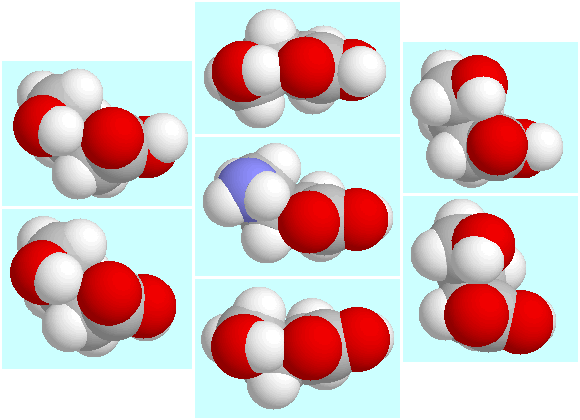
In GABA, e.g., there is a single symmetry-unique
conformer with a N-H···O=C hydrogen bond,
whereas the potential energy surface of GHB contains six symmetry-unique
conformers with the analogous O-H···O=C hydrogen bond,
which are shown below, surrounding the GABA conformer. Furthermore, GHB forms
four symmetry-unique conformers with a
O-H···(CO)O-H hydrogen bond,
which has no analogon in the GABA case.
-hydroxy
acids, the opposite is true for all other intramolecular hydrogen bonds.
In GABA, e.g., there is a single symmetry-unique
conformer with a N-H···O=C hydrogen bond,
whereas the potential energy surface of GHB contains six symmetry-unique
conformers with the analogous O-H···O=C hydrogen bond,
which are shown below, surrounding the GABA conformer. Furthermore, GHB forms
four symmetry-unique conformers with a
O-H···(CO)O-H hydrogen bond,
which has no analogon in the GABA case.
|
 -hydroxypentanoic acid.
-hydroxypentanoic acid.