Intramolecular Hydrogen Bonds in Amino Alcohols.
 |
deutsche Version. |
| © |
Copyright note. |
 |
Site-map. |
Intramolecular Hydrogen Bonds in Amino Alcohols. |
|
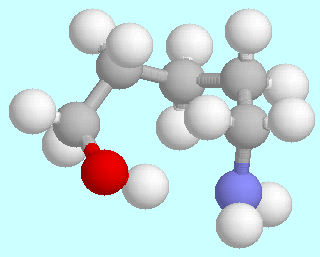
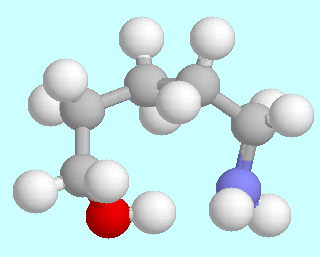
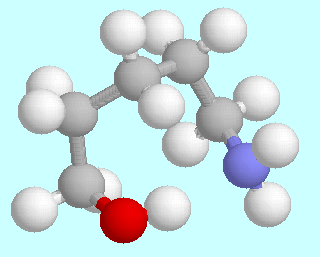
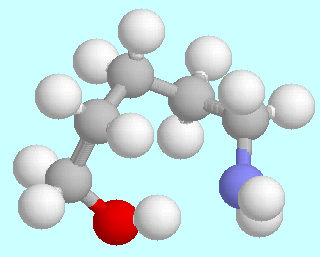
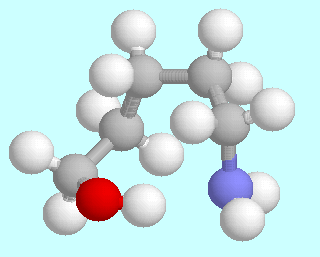
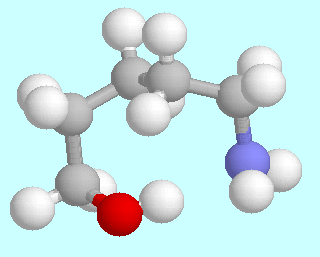
5-Aminopentanol can form six different symmetry-unique conformers that contain an O-H···N hydrogen bond. Ball-and-stick models of these conformers are shown above this paragraph, in the order of their energies. As in the amino alcohols with a shorter carbon chain, they are lowest in energy; unlike in the smaller homologous systems, there is no significant energy gap between them and the following energy minima.
Similar
to 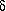 -aminopentanoic acid
and
-aminopentanoic acid
and 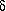 -hydroxypentanoic acid,
the various criteria for hydrogen bond strength do not give a
consistent picture in the case of the O-H···N
hydrogen bond in 5-aminopentanol:
in the global minimum, for instance, it is not only the most stable
according to energy but also according to the decrease in O-H vibration
frequency; at the same time it is least
stable according to the deviation of the O-H···N
angle from linearity,
and of intermediate strength
according to H···N
distance or increase of the O-H bond length.
A plot of the various values, that describe H-bond strength,
for the global minima of
2-aminoethanol, 3-aminopropanol, 4-aminobutanol, and 5-aminopentanol as
a function of
the ring size shows one common trend, though: an almost linear relationship
for ring-sizes up to 7 (i.e. up to 4-aminobutanol), and a significant
kink for the continuation to ring-size 8 (i.e. 5-aminopentanol).
-hydroxypentanoic acid,
the various criteria for hydrogen bond strength do not give a
consistent picture in the case of the O-H···N
hydrogen bond in 5-aminopentanol:
in the global minimum, for instance, it is not only the most stable
according to energy but also according to the decrease in O-H vibration
frequency; at the same time it is least
stable according to the deviation of the O-H···N
angle from linearity,
and of intermediate strength
according to H···N
distance or increase of the O-H bond length.
A plot of the various values, that describe H-bond strength,
for the global minima of
2-aminoethanol, 3-aminopropanol, 4-aminobutanol, and 5-aminopentanol as
a function of
the ring size shows one common trend, though: an almost linear relationship
for ring-sizes up to 7 (i.e. up to 4-aminobutanol), and a significant
kink for the continuation to ring-size 8 (i.e. 5-aminopentanol).
Besides the conformers with an O-H···N hydrogen bond, there are numerous conformers with other attractive intramolecular interactions. Most of them also exhibit repulsive H···H interactions with distances around 2.2 Å.