Intramolecular Hydrogen Bonds in Hydroxy Acids.
 |
deutsche Version. |
| © |
Copyright note. |
 |
Site-map. |
Intramolecular Hydrogen Bonds in Hydroxy Acids. |
|
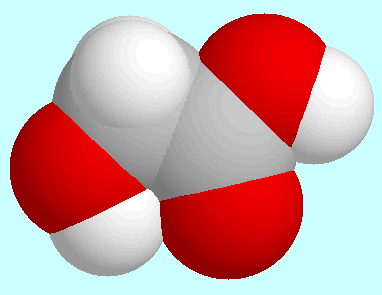 Glycolic acid
has a potential energy surface in which many stationary
points are mirror symmetrical. The global minimum, which is shown
next to this paragraph, is among these too.
It forms an intramolecular interaction
O-H···O=C with a
H···O-distance of 2.22 Å,
which, according to the electron density between the aliphatic OH- and
the CO-group, is not a hydrogen bond but of electrostatical nature.
The global minimum is quite stable: the smallest potential barrier, which
corresponds to an internal rotation of the aliphatic OH-group, is above
21 kJ/mol.
Glycolic acid
has a potential energy surface in which many stationary
points are mirror symmetrical. The global minimum, which is shown
next to this paragraph, is among these too.
It forms an intramolecular interaction
O-H···O=C with a
H···O-distance of 2.22 Å,
which, according to the electron density between the aliphatic OH- and
the CO-group, is not a hydrogen bond but of electrostatical nature.
The global minimum is quite stable: the smallest potential barrier, which
corresponds to an internal rotation of the aliphatic OH-group, is above
21 kJ/mol.
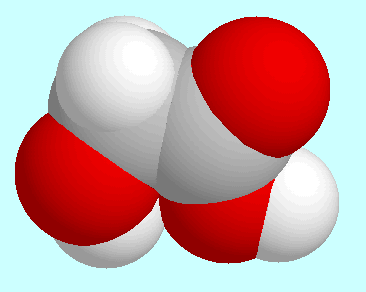 The energetically second lowest conformer, which is shown next to this
paragraph, is asymmetrical; the torsion angle
O-C
The energetically second lowest conformer, which is shown next to this
paragraph, is asymmetrical; the torsion angle
O-C -C=O
is 154°, the torsion H-O-C
-C=O
is 154°, the torsion H-O-C -C is
-44°. This conformer forms an interaction
O-H···(CO)O-H, which also
is no hydrogen bond. The same characterization is also true for the
mirror symmetrical transition state between this conformer and its
mirror image, which has been mistaken for a local minimum in early
studies [Ha et al., 1981].
-C is
-44°. This conformer forms an interaction
O-H···(CO)O-H, which also
is no hydrogen bond. The same characterization is also true for the
mirror symmetrical transition state between this conformer and its
mirror image, which has been mistaken for a local minimum in early
studies [Ha et al., 1981].
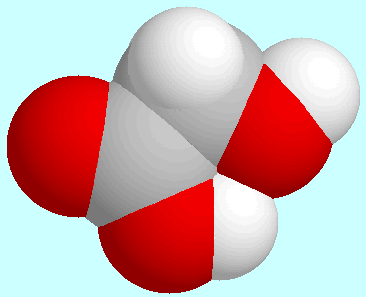 A real hydrogen bond is formed in the energetically third conformer,
which is shown next to this paragraph:
the H···O-distance is
1.97 Å, which is 75% of the sum of the
van der Waals-radii, and the O-H bond length is 0.0057 Å shorter
than in the other conformers with a trans-orientation of the
COOH group. Similarly, the calculated O-H vibration frequency is
83 cm
A real hydrogen bond is formed in the energetically third conformer,
which is shown next to this paragraph:
the H···O-distance is
1.97 Å, which is 75% of the sum of the
van der Waals-radii, and the O-H bond length is 0.0057 Å shorter
than in the other conformers with a trans-orientation of the
COOH group. Similarly, the calculated O-H vibration frequency is
83 cm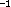 lower. This conformer is considerably stabilized by
the hydrogen bond: the smallest potential barrier is 40 kJ/mol.
lower. This conformer is considerably stabilized by
the hydrogen bond: the smallest potential barrier is 40 kJ/mol.
An interesting trend can be gathered from the deviation (square root of sum of squares of differences) of the calculated rotation constants from the experimental values for the geometries of the global minimum, which have been optimized in RHF calculations with various basis sets. Basis sets with polarization functions perform even worse than minimal basis sets in this case.
| basis | deviation | energy [a.u.] |
|---|---|---|
| STO-3G | 0.2485 | -298.63524 |
| STO-6G | 0.2066 | -301.51535 |
| 3-21G | 0.0154 | -300.96339 |
| 4-31G | 0.0816 | -302.20754 |
| 6-31G | 0.0341 | -302.51371 |
| 6-31++G | 0.0319 | -302.52575 |
| 6-311G | 0.0785 | -302.59875 |
| 6-31G* | 0.3057 | -302.65697 |
| 6-31G** | 0.3170 | -302.67352 |
| 6-31+G** | 0.3024 | -302.68443
|
 -hydroxypropionic acid.
-hydroxypropionic acid.