Intramolecular Hydrogen Bonds in Amino Aldehydes.
 |
deutsche Version. |
| © |
Copyright note. |
 |
Site-map. |
Intramolecular Hydrogen Bonds in Amino Aldehydes. |
|
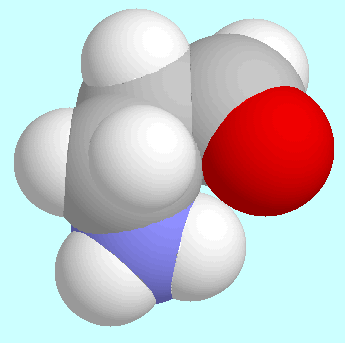 3-Aminopropanal is an interesting compound
besides the comparison with
3-Aminopropanal is an interesting compound
besides the comparison with  -alanine and 3-aminopropanol:
it simply does not exist as a stable compound.
At least there is no bibliography of experimental
results, except for a few citations of 3-aminopropanal
as an intermediate in enzymatic reactions.
This virtual non-existence of a small organic
compound, which does not pose any obvious problem either for synthesis
or for stability, is a surprising fact.
-alanine and 3-aminopropanol:
it simply does not exist as a stable compound.
At least there is no bibliography of experimental
results, except for a few citations of 3-aminopropanal
as an intermediate in enzymatic reactions.
This virtual non-existence of a small organic
compound, which does not pose any obvious problem either for synthesis
or for stability, is a surprising fact.
The results of ab initio calculations indicate an asymmetric global minimum, in which the aldehyde group is almost coplanar with the plane of the three carbon atoms. The amino group sticks out of that plane, and one amino group hydrogen atom comes close to the oxygen atom, without, however, forming a hydrogen bond.
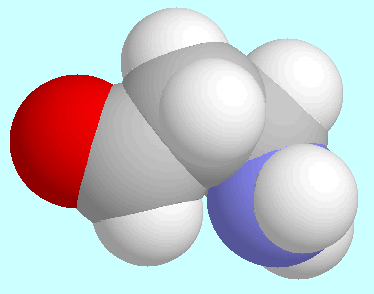 Other interesting stationary points in the potential energy surface of
3-aminopropanal are a conformer, in which the aldehyde group hydrogen atom
points towards the nitrogen atom, and a stationary point with a
horizontal tanget plane. The former is shown in the display next to
this paragraph, and is remarkable because the
C-H···N interaction
turns out to be electronically repulsive (the C-H bond length is significantly
shorter than in all other conformers, and the C-H vibration frequency
is more than 100 cm
Other interesting stationary points in the potential energy surface of
3-aminopropanal are a conformer, in which the aldehyde group hydrogen atom
points towards the nitrogen atom, and a stationary point with a
horizontal tanget plane. The former is shown in the display next to
this paragraph, and is remarkable because the
C-H···N interaction
turns out to be electronically repulsive (the C-H bond length is significantly
shorter than in all other conformers, and the C-H vibration frequency
is more than 100 cm above the average). There is, however,
enough electrostatic attraction between the positively charged
hydrogen atom and the negatively charged nitrogen atom to overcome
this repulsion.
above the average). There is, however,
enough electrostatic attraction between the positively charged
hydrogen atom and the negatively charged nitrogen atom to overcome
this repulsion.
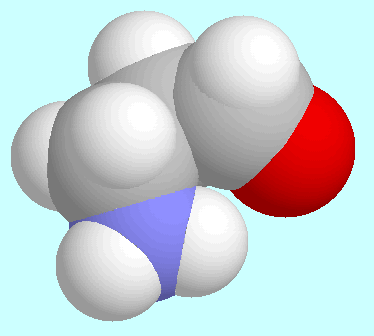 The stationary point in the potential energy surface with a
horizontal tanget plane, that is mentioned above, is shown in the
graphic insert of this paragraph. Its energy is minimal with respect
to all degrees of freedom, except the internal rotation of the
aldehyde group, for which it increases in one direction, but
decreases in the other. The harmonic frequency (although questionable
for such a potential) is 7.5 cm
The stationary point in the potential energy surface with a
horizontal tanget plane, that is mentioned above, is shown in the
graphic insert of this paragraph. Its energy is minimal with respect
to all degrees of freedom, except the internal rotation of the
aldehyde group, for which it increases in one direction, but
decreases in the other. The harmonic frequency (although questionable
for such a potential) is 7.5 cm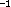 , which confirms
the flatness of the potential energy surface at this point.
(Paul Mezey
has a good comparison for such a point: it resembles
a certain type of water slide in an amusement park.) Such a point
may be considered as a conformer that has no potential barrier for
one reaction path.
, which confirms
the flatness of the potential energy surface at this point.
(Paul Mezey
has a good comparison for such a point: it resembles
a certain type of water slide in an amusement park.) Such a point
may be considered as a conformer that has no potential barrier for
one reaction path.
In the case of 3-aminopropanal this interpretation is an omen for the whole potential energy surface: there is no conformer, which has a notable kinetic stability. For each conformer there is at least one degree of freedom with a very low barrier; the kinetically most stable conformer is the global minimum with a lowest barrier of approximately 1 kcal/mol, which corresponds exactly to the thermal energy at room temperature. This seems to be the key for an explanation, why 3-aminopropanal is so unstable:
 -alanine.
-alanine.