Intramolecular Hydrogen Bonds in Amino Amides.
 |
deutsche Version. |
| © |
Copyright note. |
 |
Site-map. |
Intramolecular Hydrogen Bonds in Amino Amides. |
|
The ab initio results, which are presented along this tour, can be summarized as follows:
 The presence of a C=O group is more influential on the structures
than any intramolecular hydrogen bond.
The presence of a C=O group is more influential on the structures
than any intramolecular hydrogen bond.
 The strongest intramolecular hydrogen bond, which is formed in these
compounds, is the OC-O-H···N hydrogen bond in
The strongest intramolecular hydrogen bond, which is formed in these
compounds, is the OC-O-H···N hydrogen bond in
 -amino acids; it is
-amino acids; it is
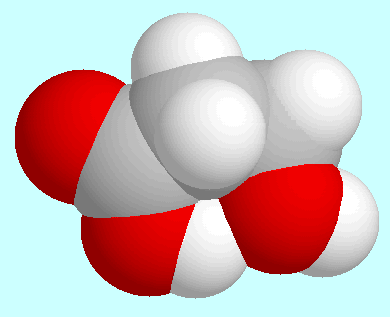
 stronger than the corresponding
OC-N-H···N bond in
stronger than the corresponding
OC-N-H···N bond in 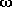 -amino amides,
-amino amides,
 stronger than the corresponding CO-O-H···O
bond in
stronger than the corresponding CO-O-H···O
bond in 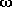 -hydroxy
acids, and
-hydroxy
acids, and
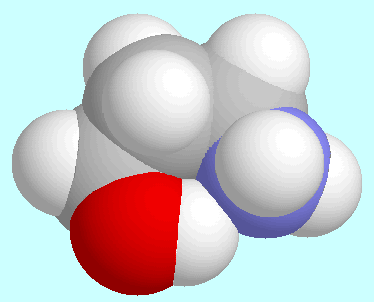 stronger than the corresponding
stronger than the corresponding
 C-O-H···N
C-O-H···N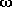 -amino-n-alkanoles.
-amino-n-alkanoles.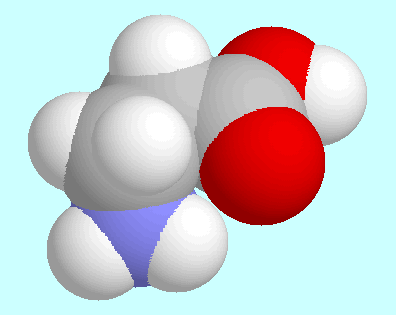 The CO-O-H···N hydrogen bond in
The CO-O-H···N hydrogen bond in
 -amino
-amino In contrast, the
corresponding O-H···O=C and
O-H···O-C=O interactions in
In contrast, the
corresponding O-H···O=C and
O-H···O-C=O interactions in
 -hydroxy
-hydroxy In 3-aminopropionamide,
the extended structures are remarkable because the internal rotation of
the
In 3-aminopropionamide,
the extended structures are remarkable because the internal rotation of
the  -group
-group -alanine shows that
sterical factors can be ruled out as an explanation for this rather
unique energy profile.
-alanine shows that
sterical factors can be ruled out as an explanation for this rather
unique energy profile.
The first three items in this summary
show impressively that amino acids are a
unique class of compounds. This is true for the interactions in the
gas-phase structures as well as for the solvated species:
in the living organism,  -amino acids
are the building blocks of
proteins and peptides; there are, e. g., no polyesters of comparable
importance. (In fact, some polyester analogues of peptides are biodegradable.)
Also, hydroxy acids do not form zwitterions in polar media,
whereas amino acids do. The stable
N···H-O-CO hydrogen bond that is
formed without competition in the gas phase, is the precursor of
this zwitterion formation, which can occur without any proton donating
solvent.
-amino acids
are the building blocks of
proteins and peptides; there are, e. g., no polyesters of comparable
importance. (In fact, some polyester analogues of peptides are biodegradable.)
Also, hydroxy acids do not form zwitterions in polar media,
whereas amino acids do. The stable
N···H-O-CO hydrogen bond that is
formed without competition in the gas phase, is the precursor of
this zwitterion formation, which can occur without any proton donating
solvent.
The last item of the above summary also has an interesting link
to peptide and protein chemistry, since it can only be explained by
assuming that the extended structure implies a special stability for the
fragment  -CO-NH-.
-CO-NH-. of a substituted version
of this very fragment in this very orientation, which generates
the sheet structure of peptides and proteins.
In the model tripeptide
of a substituted version
of this very fragment in this very orientation, which generates
the sheet structure of peptides and proteins.
In the model tripeptide  ,
,
 -alanine.
-alanine.