Intramolecular Hydrogen Bonds in Amino Amides.
 |
deutsche Version. |
| © |
Copyright note. |
 |
Site-map. |
Intramolecular Hydrogen Bonds in Amino Amides. |
|
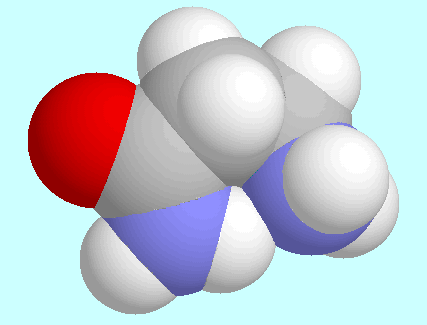
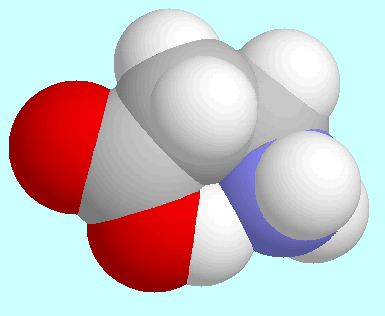 Regarding the hydrogen bonded conformers of 3-aminopropionamide
and
Regarding the hydrogen bonded conformers of 3-aminopropionamide
and  -alanine, an obvious difference is that
the former is the global minimum of the potential energy surface,
whereas the latter is not. This is due to the energetically unfavourable
trans-orientation of the COOH-group, which is a
prerequisite for hydrogen bond formation in
-alanine, an obvious difference is that
the former is the global minimum of the potential energy surface,
whereas the latter is not. This is due to the energetically unfavourable
trans-orientation of the COOH-group, which is a
prerequisite for hydrogen bond formation in  -alanine;
the stabilization of the hydrogen bond is more than cancelled by the
cis-trans-energy difference.
The CONH
-alanine;
the stabilization of the hydrogen bond is more than cancelled by the
cis-trans-energy difference.
The CONH -group, in contrast, has only one stable orientation,
so the stabilization generated by the hydrogen bond comes to effect.
-group, in contrast, has only one stable orientation,
so the stabilization generated by the hydrogen bond comes to effect.
Beyond that obvious difference, the two H···N
hydrogen bonds
are quite different in strength: N-H···N
in 3-aminopropionamide has a H···N-distance of
2.107 Å,
which corresponds to 78% of the sum of the van der Waals radii, whereas
O-H···N in  -alanine has
a H···N-distance of 1.864 Å (69%).
Similarly, the H-N bond length increases by 0.006 Å
in 3-aminopropionamide, but the O-H bond length increases by 0.017 Å
in
-alanine has
a H···N-distance of 1.864 Å (69%).
Similarly, the H-N bond length increases by 0.006 Å
in 3-aminopropionamide, but the O-H bond length increases by 0.017 Å
in  -alanine, and
the lowest potential barrier of the H-bonded conformer is
12.1 kJ/mol in the amino amide but 28.7 kJ/mol in the amino acid.
-alanine, and
the lowest potential barrier of the H-bonded conformer is
12.1 kJ/mol in the amino amide but 28.7 kJ/mol in the amino acid.
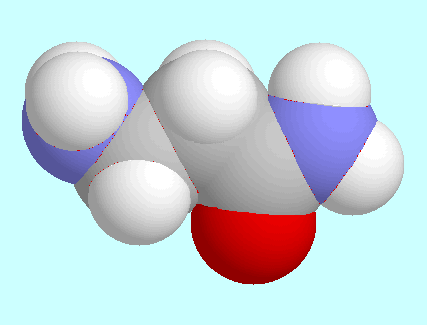 It is also noteworthy to compare the
internal rotation of the COR-group in the extended conformers.
In the 3-aminopropionamide case,
this reaction passes a single transition state after
a rotation of 180° and then leads back to the original form.
In the
It is also noteworthy to compare the
internal rotation of the COR-group in the extended conformers.
In the 3-aminopropionamide case,
this reaction passes a single transition state after
a rotation of 180° and then leads back to the original form.
In the  -alanine
case with the sterically comparable
trans-orientation of the COOH-group, three conformers
are formed along this reaction for both, symmetrical and asymmetrical
orientation of the amino group. The conformers along a full cycle of
the symmetrical extended form are shown below this paragraph.
This difference is not
simply related to steric factors
of the groups COOH and CONH
-alanine
case with the sterically comparable
trans-orientation of the COOH-group, three conformers
are formed along this reaction for both, symmetrical and asymmetrical
orientation of the amino group. The conformers along a full cycle of
the symmetrical extended form are shown below this paragraph.
This difference is not
simply related to steric factors
of the groups COOH and CONH , because the alterations of the relevant
angles are identical within fractions of a degree: in the mirror symmetrical
extended form of 3-aminopropionamide, e. g.,
the angle C-C-N is 115.74°, and in the transition
state that corresponds to this form it is 118.22°;
in the
, because the alterations of the relevant
angles are identical within fractions of a degree: in the mirror symmetrical
extended form of 3-aminopropionamide, e. g.,
the angle C-C-N is 115.74°, and in the transition
state that corresponds to this form it is 118.22°;
in the  -alanine
analogues the C-C-O angles are 115.77° and 118.39°, respectively.
-alanine
analogues the C-C-O angles are 115.77° and 118.39°, respectively.

|
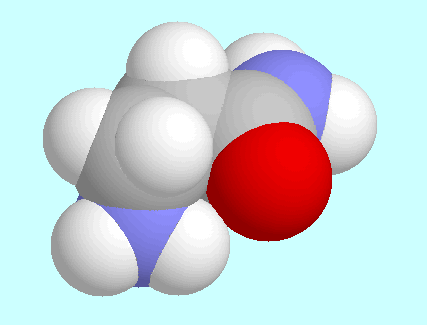
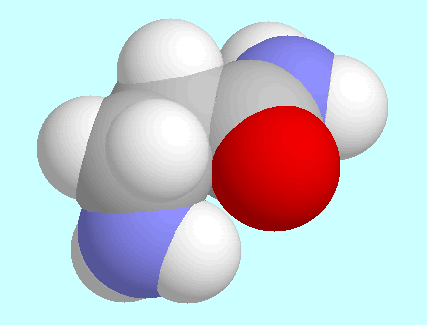 The second intramolecular interaction, N-H···O=C,
in contrast, is more or less identical in both,
3-aminopropionamide and
The second intramolecular interaction, N-H···O=C,
in contrast, is more or less identical in both,
3-aminopropionamide and  -alanine. It is no hydrogen bond,
but an attractive electrostatic interaction that has enough influence to
avoid a third orientation of the amino group. In the case of
3-aminopropionamide
it also has enough influence to couple the rotation of the groups
NH
-alanine. It is no hydrogen bond,
but an attractive electrostatic interaction that has enough influence to
avoid a third orientation of the amino group. In the case of
3-aminopropionamide
it also has enough influence to couple the rotation of the groups
NH and CONH
and CONH to a complex pattern with
three transition states, all of which toggle
between the two conformers shown next to this paragraph.
to a complex pattern with
three transition states, all of which toggle
between the two conformers shown next to this paragraph.